Stride Tech Medical, Inc. (StrideTech) set out to create a product to help older adults most vulnerable to falls. From the outset, StrideTech has known scientific and clinical proof of the efficacy of our product are vital to our mission. While the world of falls is complex and ever-changing, we know we will have done our job if we prevent even one fall. Going from measuring esoteric metrics like weight-bearing on a walker and hip-distance from a frame to clinically meaningful changes in fall risk is not simple. While COVID-19 did not make the testing processes easier, StrideTech has relentlessly continued and collected data to improve our StrideTech Go product as quickly and safely as possible. StrideTech has grown from a student project to a lean startup company on the brink of substantial growth.
Below is a summary of the data, insights, learnings, and improvements the team has made over the last three years.
Before StrideTech became a company, Tim, Humsini, and Andrew were mechanical engineering students at the University of Colorado, Boulder. Tim, Humsini, and some classmates created the first-ever StrideTech prototype in their junior year.
In a class designed to teach engineers about invention, innovation, and entrepreneurship, the team had the chance to tackle solving a problem of their choice. Having recently worked with Design for America and Medline on a walker design, Tim knew most falls are preventable, and most walker users increase their fall risk by improperly using their walker. Tim proposed designing a smart walker attachment to address preventable falls and StrideTech was born.
From ideation to the first prototype, the team’s vision was to help create a world where walker users are more mobile, more confident, and able to enjoy their friends and family, safely.
The team’s original prototype did not include feedback of any kind. Instead, a sensor counted the rotation of the wheels to estimate gait speed (a clinical indicator of fall risk), and an additional sensor measured step frequency, and by proxy, stride length (another clinical indicator of fall risk). We envisioned data would be stored and sent to physical therapists (PTs), to further inform the fall risk of a patient on a daily, rather than on an annual basis. Because the tech was designed to inform PTs of their patients' stride length, we named our hypothetical company "StrideTech".
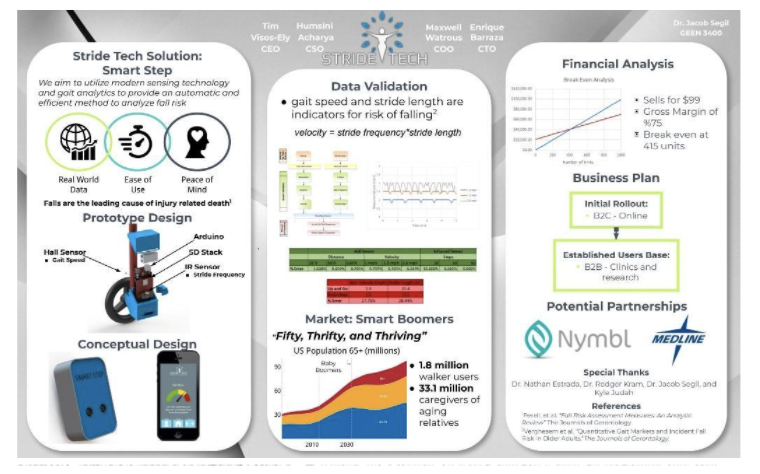
What worked: The idea and market. While StrideTech has made significant changes to the product since its inception, the fundamental idea of preventing falls before they happen has not changed.
What didn't: If someone was not stepping through the wheelbase of the walker, the step count and stride length could not accurately be measured. Additionally, measuring velocity accurately proved to be difficult due to sensor noise and the variability of walker wheels.
What we learned: While physical therapists liked the idea of a product which continually monitored fall risk, our prototype did not work effectively if the user was not using their walker correctly. Since most older adults, especially those at increased fall risk, often do not use their walkers correctly, the prototype had limited usefulness. The team had learned of a huge problem (incorrect walker use increased fall risk) but needed to find a better solution.
The team decided to pursue the smart walker idea in their senior year design capstone class. This was a yearlong design and manufacturing class every mechanical engineer is required to take in their final year of study. Instead of the more traditional industry-sponsored section, the team opted for the Engineering for Social Innovation (ESI) section. The entrepreneurial aspect of this class allowed the team to propose their problem and engineering solution to work on for the year. The team grew during this class, with the addition of Andrew Plum, who has been instrumental in the development of the mechanical and electronic aspects of StrideTech Go.
The team decided to re-design the system to better address the needs of the walker user and their PTs, who already conducted fall risk assessments and would be hesitant to trust an unproven technology. The team decided to discontinue measuring gait speed or step length, something PTs already do in fall risk assessments, and instead focus on correcting the common ways in which people incorrectly use their walkers. Based on a suggestion from our early advisor and practicing PT, Dr. Nathan Estrada, the team integrated using biofeedback to alert people to their posture into their design. After learning from Dr. Estrada about how people incorrectly use their walkers, the team focused on measuring and giving feedback on two key metrics:
The design of StrideTech GO in its current form was created. Sensors in a grip cover measured how much weight someone was putting on their walker, and a sensor on the frame measured how far away someone might be from their walker. If they exceed a safe threshold, the handles vibrate to alert them to correct their stance. At the time, the team was against adding visual feedback, thinking it could distract the user.
After designing, building, and calibrating prototypes, all that was needed was some real-world data to answer our most important question:
To find as many walker users as possible, the team hosted a "walker repair workshop" at a local senior living community. The team fixed residents' walkers for free, in the hope someone would be willing to test the fall prevention device. Their first tester used the prototype on her daily walk around the community, ensuring the prototype was tested at the same distance every day.
Over four days, the tester's number of walker misuses went from
This promising result launched the possibility this product had a real chance of helping people. Winning the University of Colorado, Boulder's New Venture Challenge competition and securing $100,000 in seed funding from the event propelled the possibility the product had commercial viability outside of the classroom and solidified the team's intent to pursue StrideTech post-graduation.

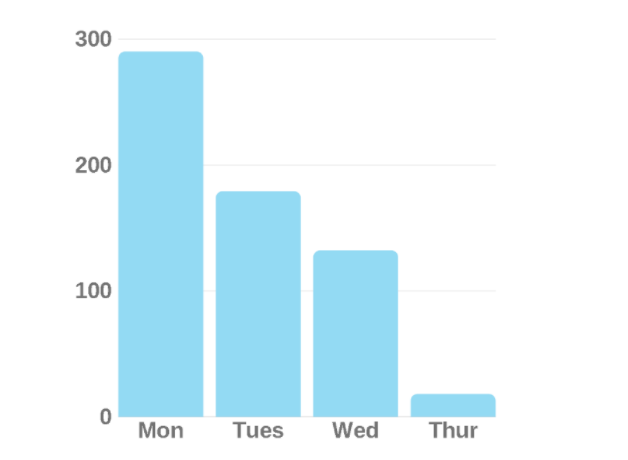
What worked: Biofeedback! Not only did we see a quantitative reduction in walker misuse, but we also discovered older adults craved anything that would help them use their walkers better.
What didn't: Our design consisted of inaccurate sensors and a grip cover design which took too long to sew and fell off too often.
What we learned: We had a promising product; we needed more product refinement, business development, and data to prove we had a viable company.
The team officially registered StrideTech Medical, Inc. on June 25th, 2019. StrideTech also began Catalyze CU – an accelerator that taught most of the business concepts essential to start-up success. The team conducted their first round of customer discovery interviews with walker users, their family members, and their health care providers. The interviews and insights from these parties formed the foundation of the user-centered design that followed. The team conducted a six-week beta test with four different residents at The Carillon at Boulder Creek, a local senior living community. The original protocol was designed to conduct one week of baseline data collection in which the team outfitted walkers with our prototype but did not provide any feedback. The team would then turn on feedback and meet weekly to download data and provide insights into walker use. The six weeks proved to serve as a much greater usability test than a definitive case study on the effects of biofeedback. Issues ranged from hand grips falling off, difficulty charging the prototypes, electrical connections breaking, and code corrupting files which contributed to a loss of data.

While little scientific data was collected from the beta test, the team learned a tremendous amount from the weekly feedback and various design failures. The experience led to a significant design change and an increase in internal testing before beta tests.
What worked: Meeting with residents weekly, observing their walker use, interviewing them about prototype usability, and understanding the stories and history behind each walker user allowed the team to have a much deeper appreciation of the potential of StrideTech GO.
What didn't: Almost every part of our design failed at one point or another: SD cards weren't storing data, electrical connections needed to be fortified, grip covers rotated 180 degrees, cases snapped, and some people gave up on testing after one week.
What we learned: Collecting data was our most important task. PTs, users, and family members all liked the idea but needed data to prove StrideTech GO worked. We had tried to do too large a beta test without conducting enough internal testing. While we learned how each component broke, and how to fix it, we lost what could have been six weeks of solid user data in the process. We learned we needed a much more robust, accurate, and reliable device first if we wanted to collect the type and amount of data needed for further validation.
Knowing the design needed to be made significantly more robust before beta testing could resume, the team spent the next six months completely overhauling the system while simultaneously developing the business plan. The team implemented sturdier wiring connectors, newer, more accurate sensors, and completely re-designed the grip covers. We sought expertise in areas such as textile design, electrical design, and firmware design.
StrideTech was simultaneously accepted into the 2020 cohort of the Boomtown HealthTech Accelerator program in Boulder, CO. The team further developed the business and met three key advisors. First, George Douaire, who mentored the team through their financial projections during the program and later joined the team full time as President and CEO. Second, Mr. Larry Blankenship, an FDA regulatory expert who subsequently joined our Board of Advisors. Third, Dr. Bradley Davidson, a human biomechanist at Denver University (DU), agreed to advise and facilitate scientific data collection, as well as co-write applications for grant funding. During their time at Boomtown, the team secured three Letters of Intent to conduct pilot testing at different senior living communities, and a plan to begin testing with DU's Human Dynamics Lab. These testing plans were critical to the team's future success and validation. Unfortunately, the emergence of SARS-COV-2 in the Spring of 2020 meant the team had to suspend user testing.
Due to the increased vulnerability of older adults, senior care communities shut down visitation from non-staff personnel. As tragic as the situation was, the increased awareness did shed a light on the need to address senior isolation and the utility of remote patient monitoring. The human dynamics lab, as well as some CU labs we had been conducting additional calibration testing with, shut down as well.
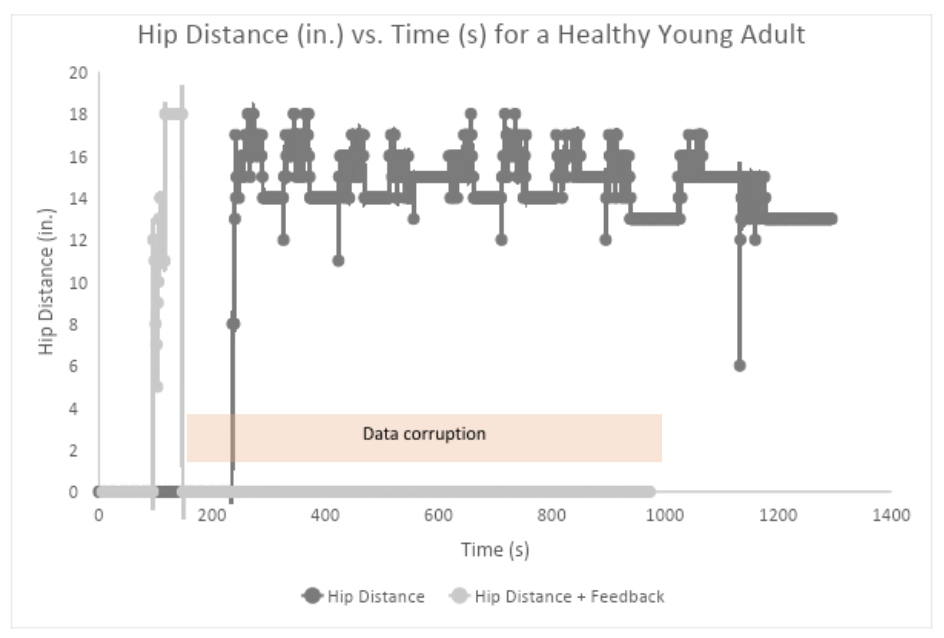
The team focused instead on internal testing and more product development. Each member of the team walked with a StrideTech GO walker for 10-15 minutes a day to characterize how metrics like weight-bearing and hip-distance would look for healthy young adults of various heights and weights.
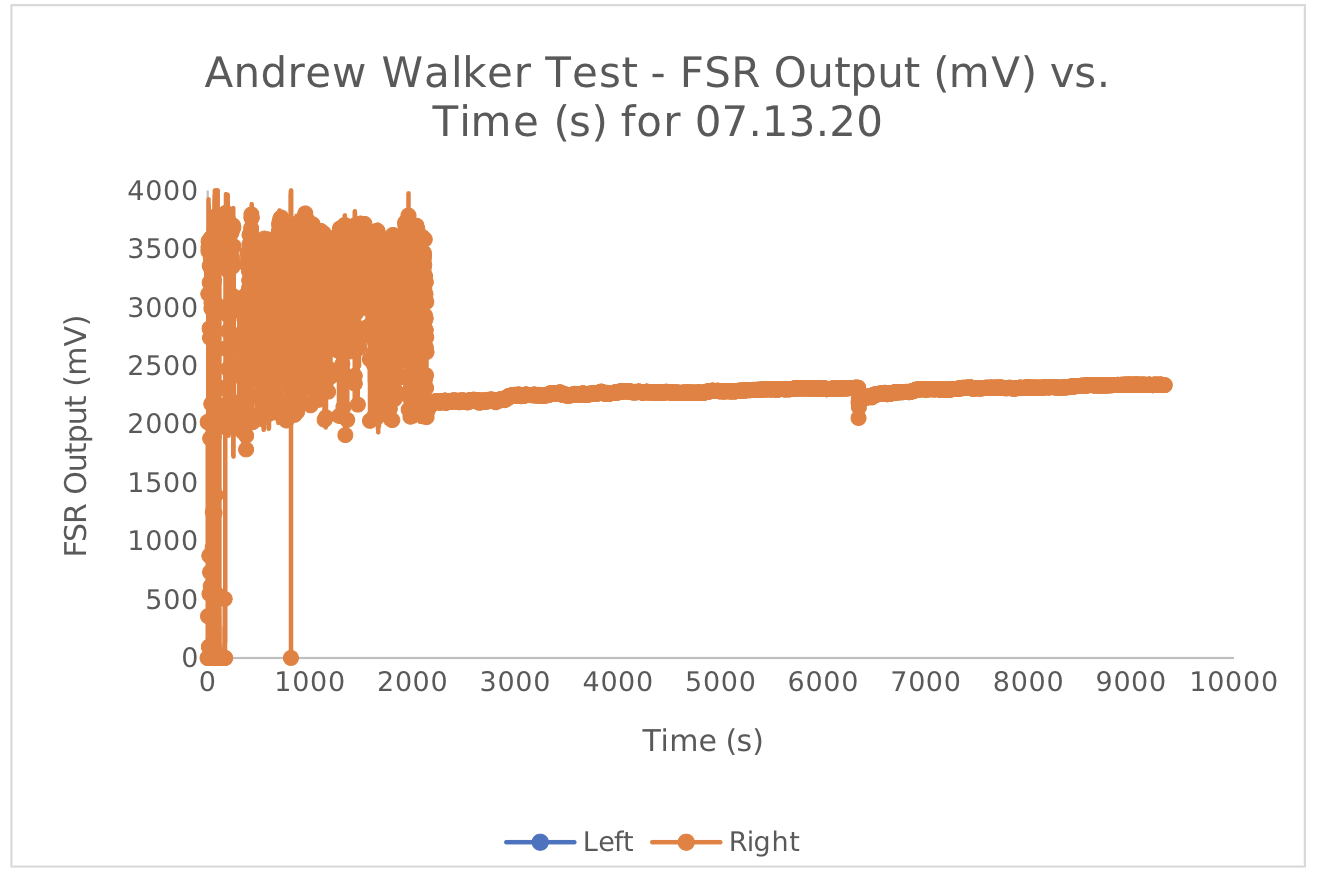

What worked: Collecting any data was better than collecting no data. Walking outside allowed the team to test with variable distances and terrains.
What didn't: There is only so much walker misuse data you can collect on three healthy young adults.
What we learned: It was difficult to feel the vibration feedback on rougher surfaces like concrete or cobblestone. We implemented a design which allowed the vibration to be turned up or down, according to preference. Importantly, we found the Time-of-Flight sensor we used to detect hip-distance gave extremely inaccurate readings when in direct sunlight. We implemented a sunshade into the enclosure designed to mitigate these effects.
As Covid cases declined, two older adult acquaintances were interested in testing with the team. We implemented COVID protocols such as masks, disinfecting walkers before and after use, and testing outdoors to mitigate risk. For a month, we would meet to walk with the tester outside for 10-15 minutes. The team iterated on data collection methods, report formats, and usability.
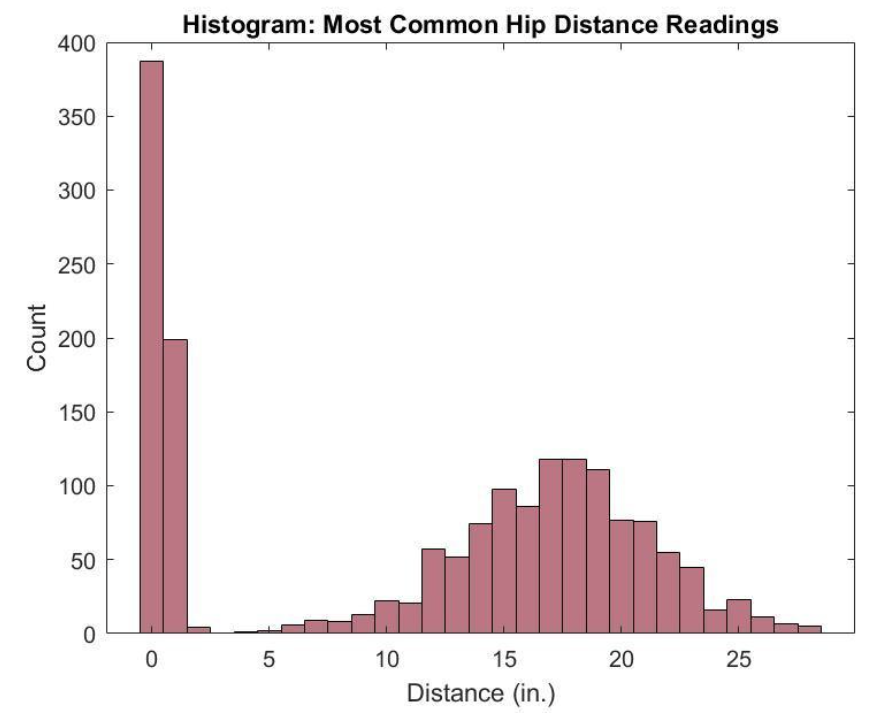
DEMONSTRATION OF ABILITY TO MEASURE ACTIVITY

What worked: Walking outdoors allowed us to observe how walkers were used "in real life". Additionally, the team was able to iterate on user and family-friendly ways to present the data collected.
What didn't: Vibration was hard to feel outside on rough or uneven terrain. Covid cases again began to spike, and testing was suspended.
What we learned: People enjoyed seeing their data week over week and were interested in intuitive measures like Activity Time, rather than how many pounds of force they were putting on their right and left handles. We integrated an LED which turned red, yellow, and green depending on the severity of misuse, to ensure feedback was communicated in situations in which it was hard to feel vibrations Importantly the more real-world walking allowed us to experience the struggle of opening and closing doors, stepping up and down curbs, and going over and around obstacles. Users were annoyed when they received vibration feedback during these movements, and we realized we needed to adjust our feedback algorithm to avoid common false positives.
To collect more data while limiting testing with older adults, the team focused instead on recruiting healthy younger adults. The team recruited seven healthy young adults to complete a set of control trials (walking with a StrideTech GO outfitted walker WITHOUT feedback) and a set of feedback trials (walking with a StrideTech GO outfitted walker WITH feedback). The purpose was to collect more walking data in a controlled indoor setting, to characterize healthy, ideal measures of weight-bearing and feedback, and assess how biofeedback may affect those measures. The team was also able to test the more rugged unit and mounting system.

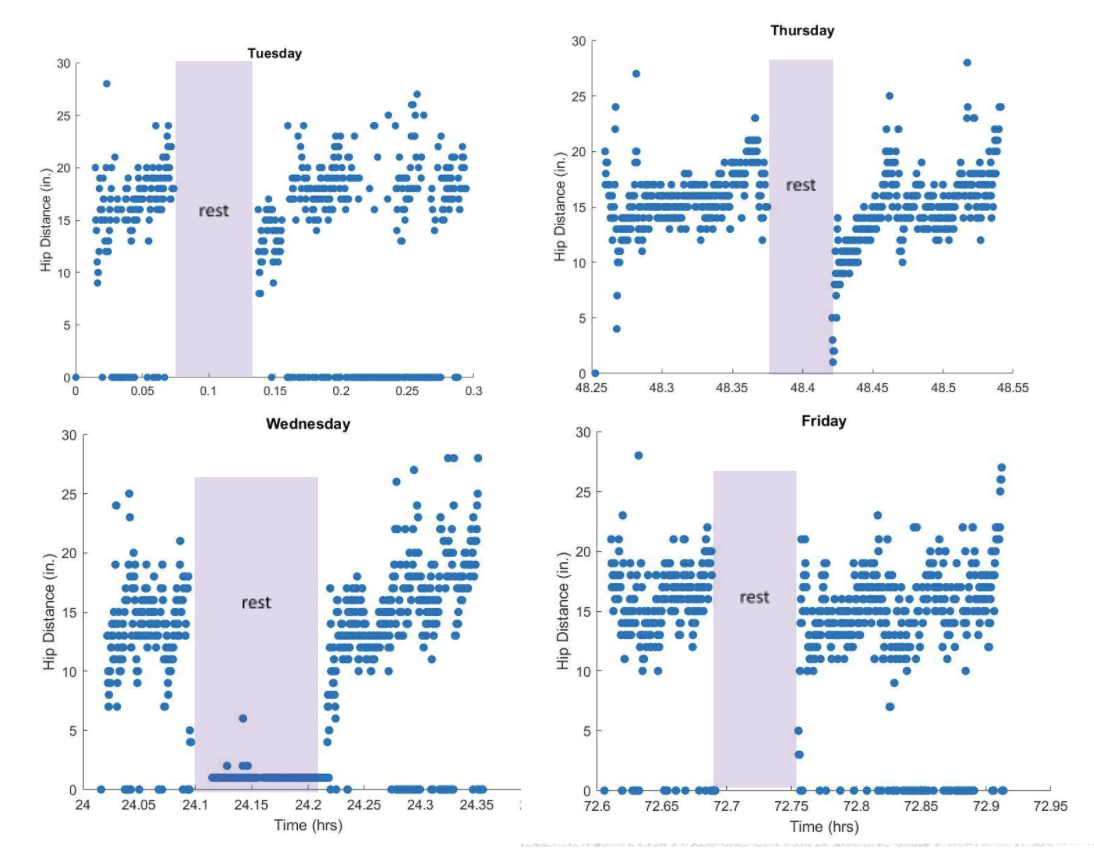
What worked: The team collected a data set of seven healthy young adults to add to our understanding of what these StrideTech GO measures look like for different populations. With senior living communities still shut down, this was a safer alternative to continue collecting data and iterating on the product.
What didn't: We did not get to test the effects of biofeedback, because the subjects were healthy enough, they never exceeded the misuse thresholds for feedback.
What we learned: We learned to hone our process for collecting large data sets simultaneously, which would later become vital as testing with seniors resumed. Additionally, we learned our current version of data collection (storing directly to an SD card, manually uploading the files) was robust enough for the type of short-term testing being conducted.
Senior care communities remained closed to non-staff; however, the team was able to conduct small characterization studies in Denver University's Human Dynamics Lab under the supervision of Dr. Davidson. The team conducted the calibration and characterization testing originally planned for the Summer of 2020. Using the lab's force plates, the team would load the walker handles from 0-100% body weight. This allowed the team to calibrate the handgrip system to known bodyweight loads, as opposed to relying solely on the calibration curve from the sensor's specifications sheet. Additionally, the team calibrated the accuracy of the hip distance sensor against the same measurement taken by the motion capture system. After calibration, the team was able to begin a repository of walker use characterization: healthy use, high hip distance, high weight-bearing, and high hip, high weight bearing use. This was done with a team member simulating these conditions in a controlled laboratory environment.
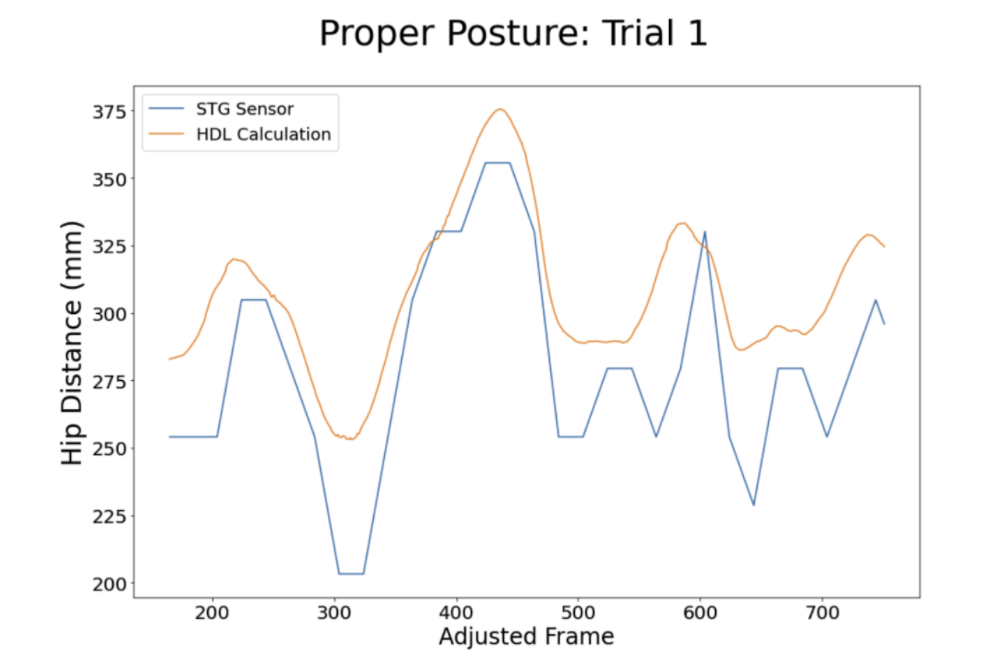

What worked: The calibration process was essential for understanding how accurate our weight-bearing and hip-distance measurements were relative to highly refined laboratory equipment. The characterization process allowed us to more clearly see what types of misuse the team might encounter and begin a repository of high-quality data.
What didn't: Simulated walker misuse is very different from natural walker misuse. The data sets produced, while highly informative and clean, were much less noisy than data coming from a walker user in the real world.
What we learned: From a scientific perspective, we first need to correlate our StrideTech GO measurements (hip distance and weight-bearing) with quantifiable and industry-standard biomechanics measures such as trunk angle, elbow angle, and balance. Improvements in clinical and research metrics combined with real user data are much more meaningful than StrideTech GO measures alone.
Vaccinations and decreases in Covid cases allowed senior care communities to begin permitting visitors again. The team resumed rollator repair shops, now adding walker disinfection to our services. Each repair shop and a group of volunteer testers further inform the team of the issues surrounding walkers and the desire for increased mobility. For these events, Tim, Andrew, and George repair and clean walkers while Humsini interviews users and tests with volunteers. Users will typically complete three control trials, then complete three feedback trials. An in-depth analysis of each of these data sets will be published in our coming White Paper series.
What worked: Older adults love trying the StrideTech GO, and constantly tell us how much they worry over how well they are using their walker. Older adults' biggest complaint and hesitancy to use a walker are that it "makes them look old". Most users know they don't want to hunch over their walker, however, most users were never instructed on how to properly use their walker in the first place. Aging adults also expressed they lose their motivation to maintain a good posture over the course of the day. Residents and staff greatly appreciated the free walker tune-up and fixes. Loose cables, broken brakes, and broken handles are common but easy fixes. The current design of the StrideTech GO units proved to be robust and no data has been lost during these testing sessions.
What didn't: We often had more people who wanted to test with us than we were able to accommodate. Most users needed further explanation on what the biofeedback means and how to correct their walker form to stop the feedback.
What we learned: Originally, we did not explain what the biofeedback was in the hope it would be intuitive. However, most users kept trying to purposefully trigger the feedback out of curiosity, which increased their walker misuses. We implemented a between-trial learning session which allowed users to first trigger weight-bearing feedback, then hip distance feedback to understand how the attachment was working. This allowed users to complete feedback trials without intentionally triggering feedback because they were trying to make sure the device was functional. As users understood what the biofeedback indicated, they tended to focus on improvements to one type of misuse (weight-bearing OR hip-distance). We hypothesize this is due to a learning effect paired with cognitive load. Additionally, we noticed users with noticeable asymmetry in weight-bearing and gait tended to always turn in one direction. Instead of a back-and-forth walking trial, we implemented a figure-eight formation to allow for data collection in straight-line walking and right and left turns. Additionally, we began recording testing sessions so qualified PTs, such as our advisor Dr. Nathan Estrada, could comment on visual walker use. More in-depth analysis and data will be provided in our White Paper series.
Barbara, a previous short-term tester at a rollator repair shop, agreed to volunteer for a six-week pilot test with us. We fitted a StrideTech GO to her walker, instructed her on how to charge the device, and asked her to use her walker as she normally would throughout the week. At weekly sessions we meet with Barbara to check-in, upload the past week's data, and show her walker use data from the week prior.

What worked: Barbara has been a fantastic long-term tester. She frequently travels via Uber (which allows us to assess how durable the StrideTech GO is when it is folded and loaded into a car), walks in nearby malls and parks, and is very honest about what does or doesn't work for her. She likes the magnetic charger because it is much easier to use than a traditional charger. She cares about her posture but says she knows she pays less attention to it throughout the day. So far, she has said StrideTech GO has been great, reminding her not to slouch, and not to press into the handles, which she had not realized she was doing. The weekly check-ins have allowed us to fix problems as they arise and iterate on ways to display data. Additionally, Dr. Brennan Severs, a PT working in Modena Cherry Creek Senior Living, partnered with us to administer fall risk assessments throughout the testing process and compare results.
What didn't: Barbara uses an oxygen machine. She typically hangs her oxygen case on the handles so it is out of the way and she can sit in the walker seat easily. With the StrideTech GO device on her walker, she had to put her oxygen case on her walker seat. Any time she had to sit down, she had to move the oxygen case, which became cumbersome and potentially dangerous if she was tired. Additionally, though the hip distance sensor is designed to be swiveled out of the way when a user needs to use their walker seat, we have noticed it is frequently put back in the wrong position. To accommodate the use of Barbara's oxygen case, we have provided hooks which enable Barbara unimpeded use of her walker seat. To accommodate returning StrideTech GO device to its proper placement on the walker, we have provided visual checkmarks (e.g., markings on the mounting system) to ensure the device is easily remounted properly. We have had a few PTs and users inquire about the availability of testing units. StrideTech is in the process of raising funds to build additional units to enable more testing and retail sales of StrideTech GO units.
What we learned: To address the issues around Barbara’s oxygen case, the team provided her with walker hooks to hang her case on, away from StrideTech GO connection points. As of 04/14/22, we are awaiting feedback to determine if the hooks have resolved the oxygen case placement issue. We additionally learned Barbara is also swiveling the hip sensor case to see the LED more easily. This is leading us to a case redesign that centers the LED on the case side facing the user, rather than the case side lateral to the user. StrideTech will develop a simple app which allows easy threshold adjustment to facilitate setup and changes to thresholds as users progress and learn to improve their walker usage.
As we move forward, StrideTech will continue to collected data to improve our StrideTech Go product as quickly and safely as possible. We invite you to join us in the future of mobility by investing in StrideTech today.